Cerapedics Announces FDA Approval of PearlMatrix™ P-15 Peptide Enhanced Bone Graft, The First and Only Proven Bone Growth Accelerator for Lumbar Fusion
OrthoSpineNews
JUNE 23, 2025
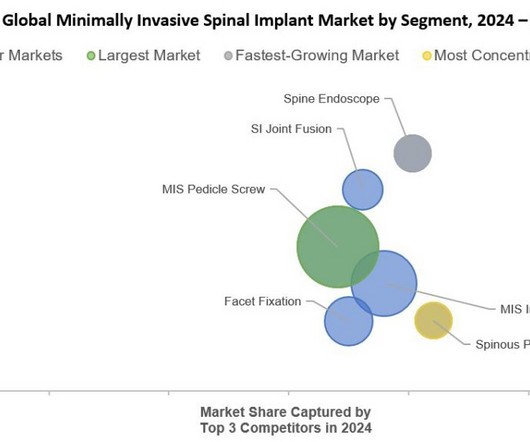
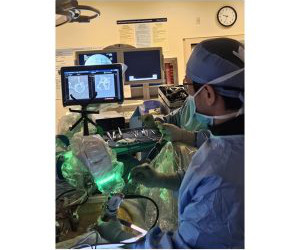
Food and Drug Administration (FDA) premarket approval (PMA) of PearlMatrix P-15 Peptide Enhanced Bone Graft as a Class III drug-device combination product for use in single-level transforaminal lumbar interbody fusion (TLIF) surgery in adult patients with degenerative disc disease (DDD).







Let's personalize your content