Things To Avoid With Degenerative Disc Disease
Oviedo Chiropractic
MARCH 25, 2024
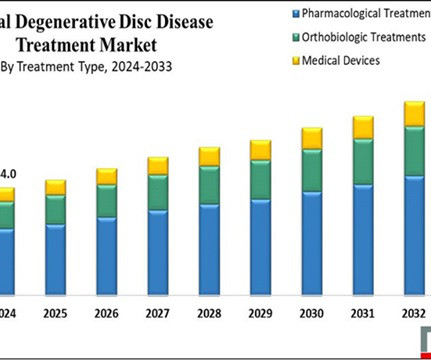
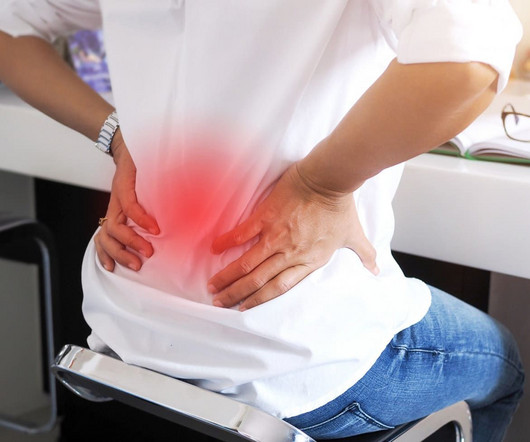
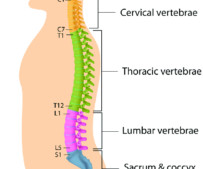
Here are some things to avoid with degenerative disc disease. However, for those living with degenerative disc disease, this process generally happens earlier—and causes more pain. If you’ve recently been diagnosed with disc degeneration, you’re probably wondering what to expect.



































Let's personalize your content