ReGelTec Announces First Patient Treated in U.S. IDE Clinical Trial for HYDRAFIL System to Treat Chronic Low Back Pain
OrthoSpineNews
JUNE 18, 2025
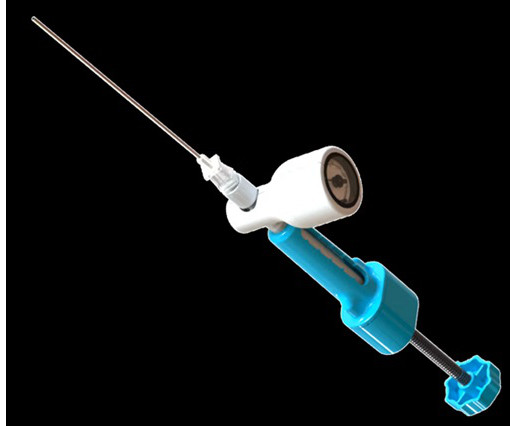
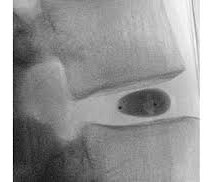
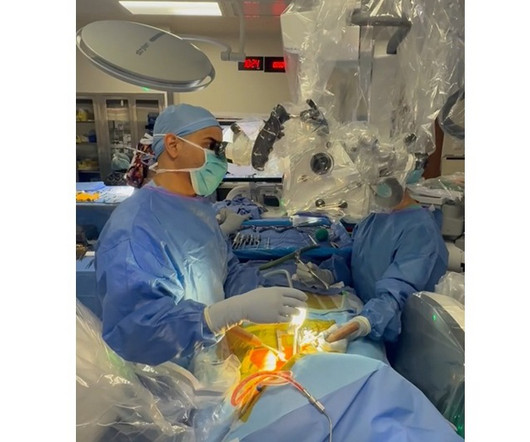
BALTIMORE, June 18, 2025 -( BUSINESS WIRE )- ReGelTec Inc. , study follows the company’s receipt of CE Mark for the HYDRAFIL System based on promising results of clinical studies performed outside the United States on 75 patients. The launch of the HYDRAFIL-D U.S. Walnut Creek, Calif.; Greenwood Village, Colo.; Jasper, Ga.;












Let's personalize your content