ReGelTec Announces First Patient Treated in U.S. IDE Clinical Trial for HYDRAFIL System to Treat Chronic Low Back Pain
OrthoSpineNews
JUNE 18, 2025
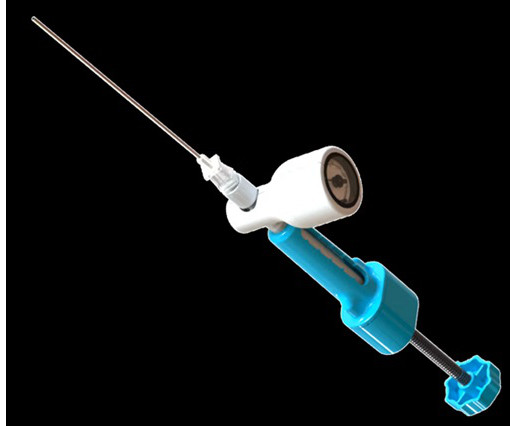
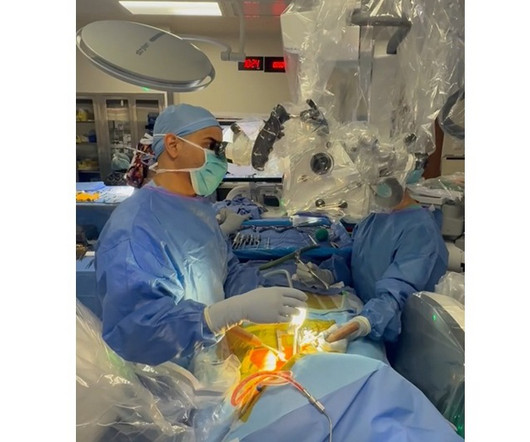
BALTIMORE, June 18, 2025 -( BUSINESS WIRE )- ReGelTec Inc. , I am excited to be working with ReGelTec and the other principal investigators to enroll the HYDRAFIL-D Study and evaluate the impact of HYDRAFIL on patients with degenerative disc disease.” Walnut Creek, Calif.; Greenwood Village, Colo.; Jasper, Ga.;













Let's personalize your content