Groundbreaking Spine Surgery Performed by Dr. Ehsan Jazini Marks New Era in Motion-Preserving Procedures
OrthoSpineNews
JUNE 25, 2025
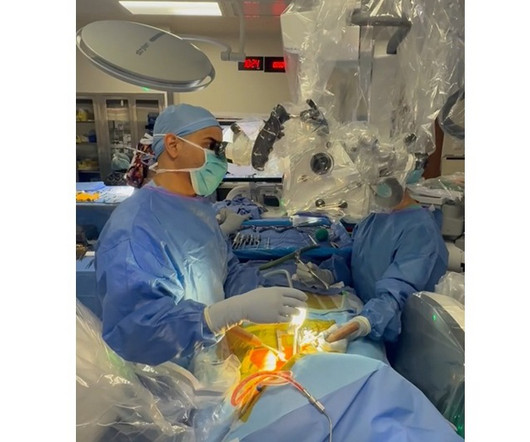
First in the Nation to Perform Custom Combination of prodisc® C Nova and prodisc® C VIVO Disc Replacement. June 25, 2025 /PRNewswire/ — VSI® proudly announces a groundbreaking achievement in spine surgery, led by nationally recognized spine surgeon Dr. Ehsan Jazini. RESTON, Va.,






Let's personalize your content