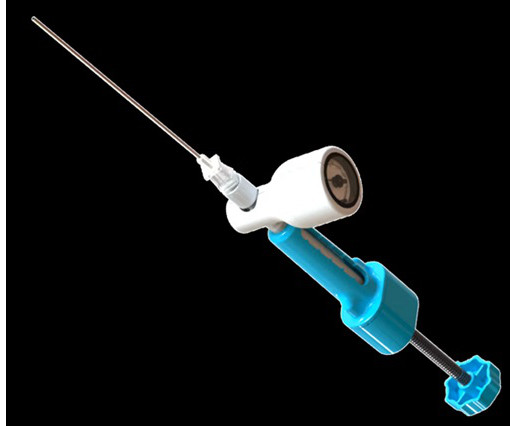
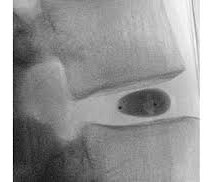
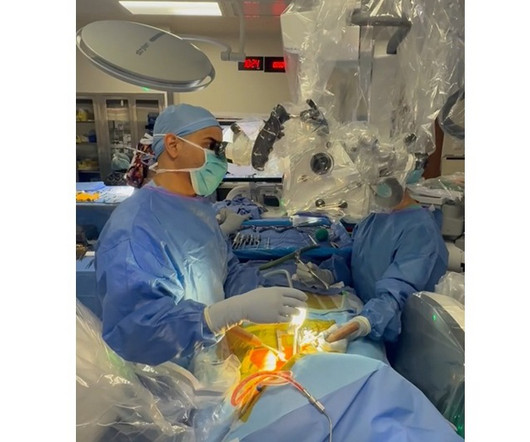
ReGelTec Announces First Patient Treated in U.S. IDE Clinical Trial for HYDRAFIL System to Treat Chronic Low Back Pain
OrthoSpineNews
JUNE 18, 2025
BALTIMORE, June 18, 2025 -( BUSINESS WIRE )- ReGelTec Inc. , BALTIMORE, June 18, 2025 -( BUSINESS WIRE )- ReGelTec Inc. , The patient was enrolled by Kas Amirdelfan, M.D., The protocol includes an interim safety analysis when the first sixty patients complete their six-month follow-up visit. in Walnut Creek, Calif.













Let's personalize your content