ReGelTec Announces First Patient Treated in U.S. IDE Clinical Trial for HYDRAFIL System to Treat Chronic Low Back Pain
OrthoSpineNews
JUNE 18, 2025
BALTIMORE, June 18, 2025 -( BUSINESS WIRE )- ReGelTec Inc. , An overwhelming majority of my chronic low back pain patients either do not want or are not good candidates for invasive spine surgeries, yet they continue to feel debilitated, without many treatment options,” said Dr. Amirdelfan. Walnut Creek, Calif.; Jasper, Ga.;

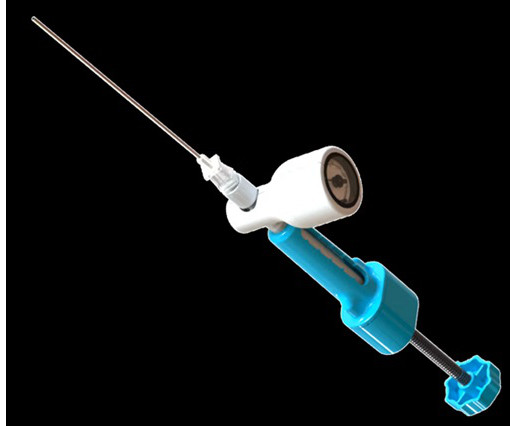


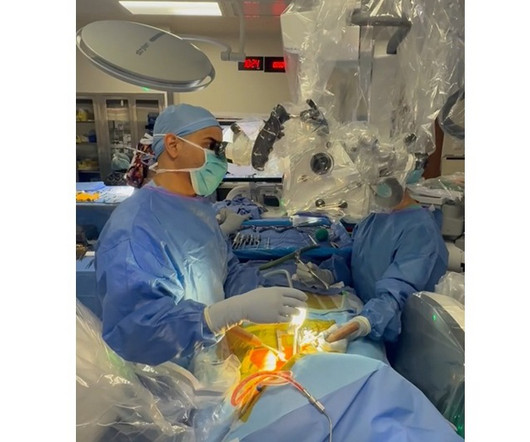
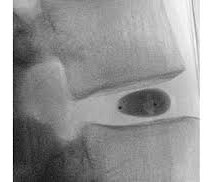










Let's personalize your content