Phase 2 Trial of BRTX-100 in cLDD Continues to Generate Positive Preliminary Blinded Data
OrthoSpineNews
MAY 13, 2025
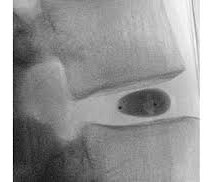
No serious adverse events (SAEs) were reported, and there was no dose (40X10 6 cells) limiting toxicity at 26-104 weeks. We have commenced a Phase 2 clinical trial using BRTX-100 to treat chronic lower back pain arising from degenerative disc disease.











Let's personalize your content