ReGelTec Announces First Patient Treated in U.S. IDE Clinical Trial for HYDRAFIL System to Treat Chronic Low Back Pain
OrthoSpineNews
JUNE 18, 2025
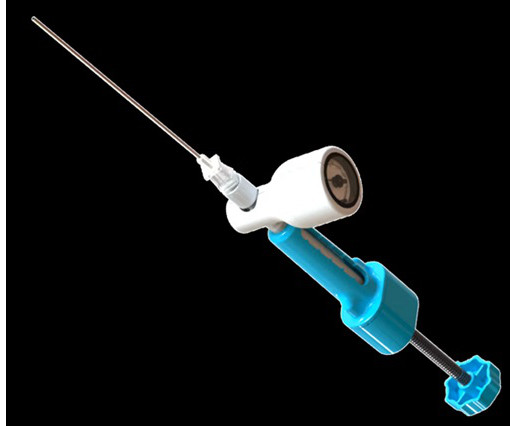
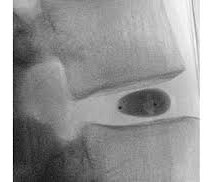
a company developing a percutaneous hydrogel implant for the treatment of chronic low back pain caused by degenerative disc disease, announced it has treated the first patient in its HYDRAFIL-D FDA investigational device exemption (IDE) clinical trial for its HYDRAFIL ® System for disc augmentation. Jasper, Ga.;


























Let's personalize your content