BioRestorative Therapies Reports First Quarter 2025 Financial Results and Provides Business Update
OrthoSpineNews
MAY 14, 2025
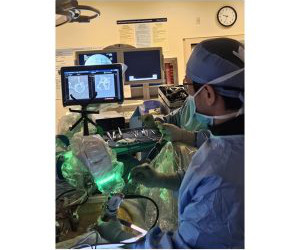
On the heels of granting BRTX-100 Fast Track designation for cLDD, the FDA cleared the Companys Investigational New Drug (IND) application for BRTX-100 for the treatment of chronic cervical discogenic pain (cCDP), expanding BioRestoratives advanced clinical pipeline for BRTX-100 to include treatment of both chronic lower back and neck pain.



Let's personalize your content