OrthoPediatrics Corp. Expands Scoliosis Portfolio with Launch of VerteGlide™ System
OrthoSpineNews
APRIL 8, 2025
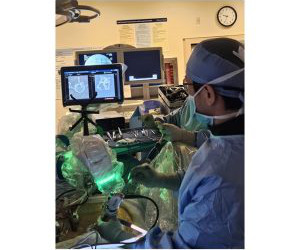
April 08, 2025 (GLOBE NEWSWIRE) — OrthoPediatrics Corp. launch of the new VerteGlide Spinal Growth Guidance System, used to treat Early Onset Scoliosis (EOS). ” OrthoPediatrics Scoliosis division President, Greg Odle commented We are excited to introduce another new product to treat children with EOS. WARSAW, Ind.,
















Let's personalize your content